At CHCT, we strive to provide services ahead of the industry times.
IRB Submission and Approval
Under FDA regulations, an Institutional Review Board is group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection […]
Read morePatient Followup
A process involving periodic contact with the patient after enrollment into the trial for the purpose of administering the assigned treatment, observing the effects of treatment, modifying the course of treatment, and collecting data to evaluate the treatment.
Read moreCompliance Patient Counselling
Are your patients compliant, and is the data you’re collecting reliable? Are you going to need to enroll more patients due to uncertainty? If you’re conducting research, you might be encountering these questions right now. If you’re about to start a study, you should anticipate these questions. Sponsors deal with myriad considerations in how to […]
Read moreContract Negotiations with Clinical Trial Sponsor
For each clinical trial, a clinical trial agreement (CTA) and budget are negotiated between the investigator and the sponsoring company so that the costs of carrying out the trial are reimbursed. Negotiation is a part of everyday life, especially in clinical trials. Many professionals and lawyers spend hours daily negotiating. However, few people have […]
Read morePatient Recruitment
Achieve your clinical trial patient enrollment goals with our proven patient-first strategic approaches to patient recruitment. Patient recruitment delays are remarkably common and costly. According to Clinical Leader, nearly 80 percent of patient recruitment timelines in clinical trials are not met and over 50 percent of the patients are not enrolled within the planned […]
Read moreSite Selection
What is Site Selection in Clinical Trials? Site selection in clinical trials refers to the process of identifying and choosing the appropriate research sites where the study will be conducted. Site selection aims to ensure that the trial is executed in a way that is ethical, safe, and compliant with all applicable regulations. A […]
Read moreSite Monitoring Visits
Site monitoring visits are to ensure compliance with protocol, Good Clinical Practice, Federal and local regulations and SOP’s. Regular site monitoring visits can be pre-study visits, initiation visits, periodic monitoring visits, and close-out visits. Study sites may also be monitored or audited by the FDA, Clinical Research Organizations (CROs), IRBs and sponsors.
Read moreProtocol Management
Clinical Protocol Management provides support for the infrastructural elements of clinical research. It includes Protocol-related activities that are completed to manage the protocol like IRB submission, Budget preparation and negotiation, Coordinating pharmacy review and set-up and Financial review and close-out.
Read moreSite Initiation
Site initiation is the final step in the study process prior to site activation. Sponsor or Clinical research organization conduct the site initiation visit after the institutional Review Board approves the clinical research study at the site and the clinical research study agreement is signed. The visit serves to train staff on the protocol […]
Read moreTrial Closeout Operation
Clinical Trial Close-out is the act of ensuring that all clinical trial related activities are appropriately reconciled, recorded and reported at the end of a trial in accordance with the protocol, SOPs, GCP, and the applicable regulatory requirement(s). Usually the checklist for this visit includes 1. Review the project scope and ensure all activities […]
Read moreReporting Adverse Events to the sponsor/CRO/IRB/IEC
Investigators are required to report promptly to the sponsor any adverse effect that may reasonably be regarded as caused by, or probably caused by, the drug. If the adverse effect is alarming, the investigator shall report the adverse effect immediately. serious adverse events should be reported to the IRB within one week of the […]
Read moreSite Services
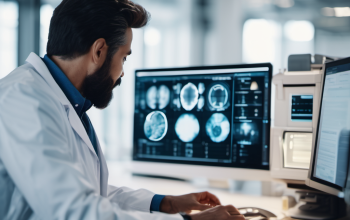
Diagnostic Imaging
At Cardio Health, Medical Imaging Department is providing trust worthy services by taking images of the body using different technique. The images are used for clinical diagnosis, treatment or disease monitoring. These modalities are Computed Tomography, Magnetic Resonance Imaging, Ultrasound and X-ray.
Read moreColonoscopy
Our expert team at Cardio Health is proud to offer a modern outpatient clinic providing Colonoscopy in a safe and comfortable environment. A colonoscopy is an examination of the inside of your large intestine. Its helpful for diagnosing gastrointestinal diseases such as inflammatory bowel disease and colon cancer.
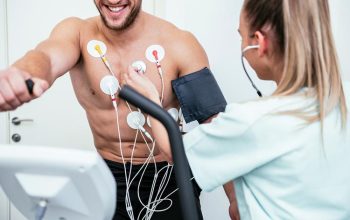
Read moreStress Echo
A collective diagnostic test which consists of a 2 phase Echocardiogram measuring your heart at a resting rate and a working rate which is achieved by the method of maximizing your heart rate via exercising in various stages on a treadmill till a target heart rate is achieved.
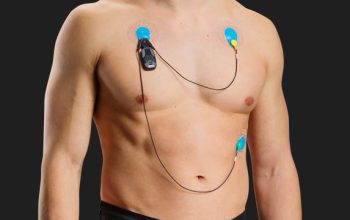
Read moreHolter Monitoring
Continuous Heart monitoring in durations of 24, 48, 72hrs and 2 weeks, results are downloaded, analyzed and reviewed by a Cardiologist.
Read moreProtocol Management
Clinical Protocol Management provides support for the infrastructural elements of clinical research. It includes Protocol-related activities that are completed to manage the protocol like IRB submission, Budget preparation and negotiation, Coordinating pharmacy review and set-up and Financial review and close-out.
Read more